Electron Paramagnetic Resonance (EPR) technology offers solutions for water treatment research
The main pollutants in water bodies include pharmaceuticals, surfactants, personal care products, synthetic dyes, pesticides, and industrial chemicals. These pollutants are challenging to remove and can adversely affect human health, including the nervous, developmental, and reproductive systems. Therefore, protecting water environments is of utmost importance.
In recent years, advanced oxidation processes (AOPs) such as Fenton-like reactions, persulfate activation, and UV-light-induced AOPs (e.g., UV/Cl2, UV/NH2Cl, UV/H2O2, UV/PS) as well as photocatalysts (e.g., bismuth vanadate (BiVO4), bismuth tungstate (Bi2WO6), carbon nitride (C3N4), titanium dioxide (TiO2) have gained attention in the field of water treatment and environmental remediation.
These systems can generate highly reactive species such as hydroxyl radicals (•OH), sulfate radicals (•SO4-), superoxide radicals (•O2-), singlet oxygen (1O2), etc. These techniques significantly enhance the removal rates of organic pollutants compared to conventional physical and biological methods. The development of these water treatment technologies greatly benefits from the assistance of Electron Paramagnetic Resonance (EPR) technology.
CIQTEK offers the desktop Electron Paramagnetic Resonance spectrometer EPR200M and the X-band continuous-wave Electron Paramagnetic Resonance spectrometer EPR200-Plus, which provide solutions for studying photocatalysis and advanced oxidation processes in water treatment.
Application Solutions of Electron Paramagnetic Resonance (EPR) technology in water treatment research
- Detect, identify, and quantify reactive species such as •OH, •SO4-, •O2-, 1O2, and other active species generated in photocatalytic and AOPs systems.
- Detect and quantify vacancies/defects in remediation materials, such as oxygen vacancies, nitrogen vacancies, sulfur vacancies, etc.
- Detect doped transition metals in catalytic materials.
- Verify the feasibility and assist in optimizing various parameters of water treatment processes.
- Detect and determine the proportion of reactive species during water treatment processes, providing direct evidence for pollutant degradation mechanisms.

Application Cases of Electron Paramagnetic Resonance (EPR) technology in water treatment research
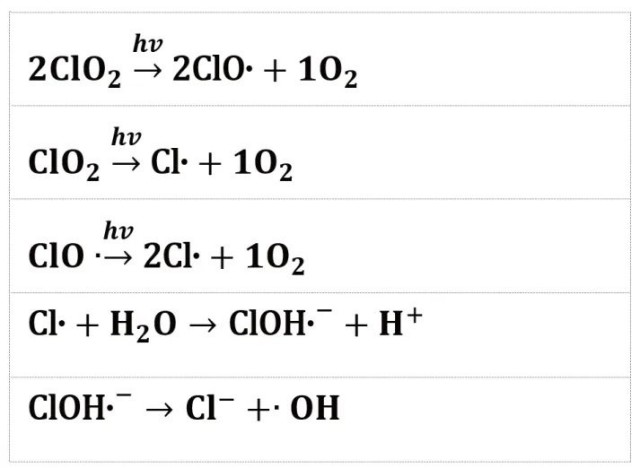
Case 1: EPR in UV/ClO2-based advanced oxidation technology
- EPR study of the degradation process of fluoroquinolone antibiotics in a UV-mediated AOPs system.
- Degradation of pharmaceuticals and personal care products (PPCPs) in water by chlorine dioxide under UV conditions.
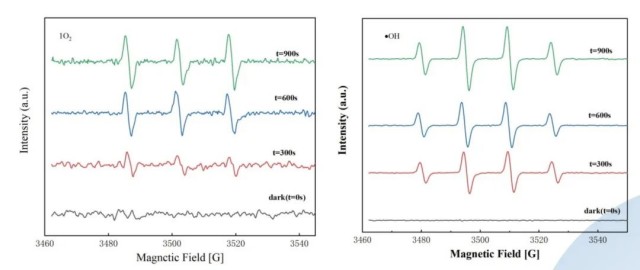
- EPR detection and qualitative analysis of •OH and singlet oxygen as active species in the system.
- - Increase in •OH and 1O2 concentrations with longer irradiation times, promoting antibiotic degradation.
- EPR detection of •OH and 1O2 concentrations can be used to optimize PPCPs treatment processes.
Case 2: EPR in Fenton-like advanced oxidation technology
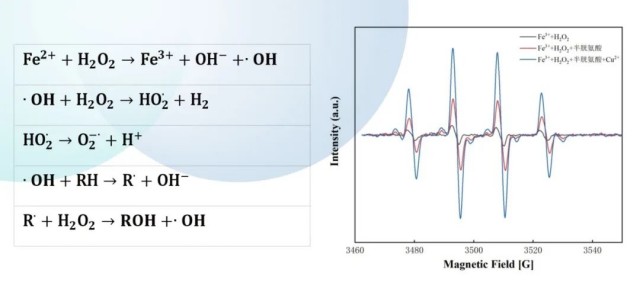
- - Degradation of phenylurea herbicides (e.g., isoproturon, linuron) in water by Fenton-like reactions.
- - EPR detection, identification, and quantification of all reactive species in the system.
- -EPR-assisted optimization of radical generation rates, such as pH, hydrogen peroxide concentration, transition metal ions, etc.
-Increased hydroxyl radical concentrations and enhanced pollutant degradation with the addition of cysteine and Cu2+.
-EPR detection and quantification of hydroxyl radicals can directly validate the herbicide degradation mechanisms.
Case 3: EPR in TiO2-based photocatalysis technology
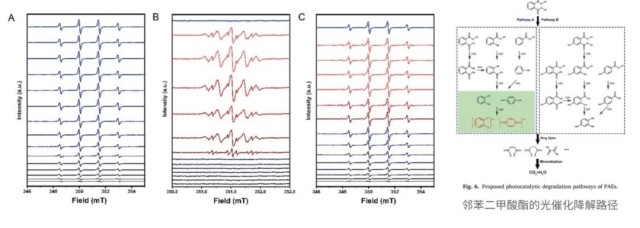
- -In situ EPR reveals the promotion of phthalic acid esters (PAEs) degradation and mineralization in water by PAEs-TiO2, resulting in nearly 100% detoxification of water.
- -In situ EPR detection of intermediate radicals, such as •OH and PAEs' semiquinone anions, during the opening process of PAEs.
- -Combined in situ EPR and quantitative analysis suggest a higher level of opening formation on selective PAEs-TiO2 photocatalysts.
-Combining in situ EPR with UPLC-QTOF/MS provides insights into the degradation pathways of PAEs on PAEs-TiO2 and TiO2 photocatalysts.
Photocatalysis technology mainly relies on the absorption of solar energy by semiconductor photocatalysts, which generates separated photoinduced electrons and holes. These reactive species, such as •OH and •O2-, formed on the surface of the photocatalyst, can degrade pollutants through processes such as addition, substitution, and electron transfer.
Moreover, these techniques efficiently and broadly disinfect and sterilize water, leading to water purification and environmental restoration. The self-developed Electron Paramagnetic Resonance Spectrometer by CIQTEK can provide solutions for studying the mechanisms of reactive species, pollutant degradation pathways, and active sites of catalysts involved in water treatment processes.
